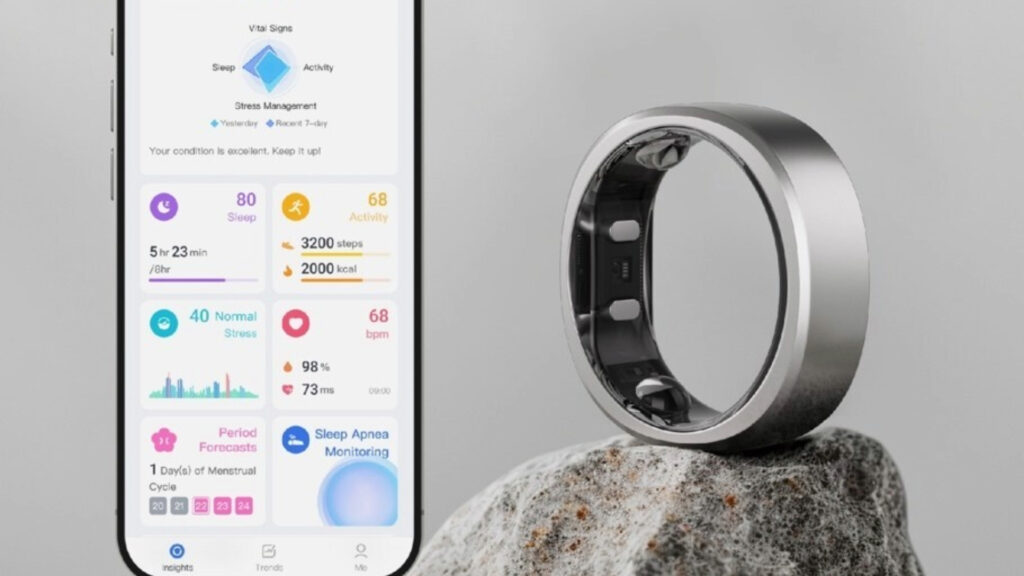
RingConn, a wearable technology startup founded in 2021, has announced the launch of its Gen 2 Smart Ring. RingConn’s new device boasts an extended battery life of 10-12 days. It’s now available for pre-order via Kickstarter, a crowdfunding platform. The company promotes this device as the first smart ring with integrated sleep apnea detection; however, some users of the popular smart ring made by Oura Health have also spotted indicators of sleep disorders in their data.
Key Points
- Innovative Health Monitoring: The Gen 2 Smart Ring introduces built-in sleep apnea monitoring, which the company describes as a pre-diagnostic tool and continuous screening method.
- Extended Battery Life: Wearables aren’t just about integrated features; they must be integrated into consumer lifestyles. The 10-12 days of battery life, with a charging case extending usage to over 150 days, could help make this more feasible.
- Compact and Lightweight Design: The ring is also exceptionally thin and light, with a minimum thickness of 2mm and a weight of just 2 grams for the smallest size.
- Advanced Features: The available range of digital health metrics includes detailed sleep analysis, exercise insights, stress monitoring, heart rate and blood oxygen tracking, and menstrual cycle predictions for female users.
- Research and Development: RingConn says the device is the culmination of extensive clinical research. They are now pursuing FDA clearance for the sleep apnea monitoring feature.
While the Gen 2 Smart Ring represents a way to make digital health more accessible and less intrusive, it’s essential to recognize that it does not replace professional medical diagnosis or treatment.
The Data
- An estimated 936 million adults (aged 30-69) have sleep apnea, with 425 million suffering from moderate to severe conditions.
- RingConn’s experimental results suggest that their algorithm can identify sleep apnea with a 90.7% accuracy rate.
- The company also claims to have “set a new standard in smart ring battery life,” doubling the endurance of competitive devices.
Industry Context
Digital tools are giving providers a more holistic view of patient health.
The FDA
Wearable devices enable new capabilities and efficiencies across digital health. Nevertheless, many physicians remain skeptical of data from consumer wearables for various reasons, such as opaque or black box algorithms, the entanglement of commercial interests with medical research, and the potential for misleading data that may prompt expensive tests or distress patients.